Of These Ph's Which Is the Best Buffer
Answer 1 of 3. Once accomplished properly this provides you a solution that you could utilize for the pH 7 buffer.
Gently add potassium hydroxide in order to bring the pH by up to 7.

. You have a 2500-mL sample of 100 M acetic acid Ka 18 10-5. Both c and d give you pH 926 since baseacid 1 and log 1 0. A good buffer is 1 of the pKa so the best buffer is acetic acid with a pka of 476.
Formic acid pK a 38 acetic acid pK a 476 or ethylamine pK a 90. Briefly justify your answer. But d is the better answer because the more concentrated solutions make the buffer capacity larger.
20 mM Na2HPO4 10 ACN in water pH 95 Solvent B. There is a wide variety of compositions that can be used to produce a buffer solution An acid buffer is produced from mixing solutions of a weak acid and the conjugate base of the acid. Buffer solutions resist any change in pH.
A good buffer is 1 of the p Ka so the best buffer is acetic acid with a pka of 476. While the lungs keep CO2 levels in check the kidneys are responsible for monitoring the amount of HCO3- or bicarbonate by excreting it or in most cases reabsorbing it. The one with the smallest pH change is the best buffer.
A buffer solution contains a mixture of a weak acid and its conjugate base or vice versa. Formic acid pKa 38 acetic acid pKa476 or ethylamine pKa 90. Depending on what pH you want though it may be better to use a certain one.
An alternative pH buffer for pH 35 is the phosphate buffer that can be obtained by dissolving 680 g of potassium dihydrogen. Briefly justify your answer. The pH value of 7 is taken as neutral less than 7 is acidic and more than 7 is basic.
A solution of HCIO and Nacio e. Meanwhile for phosphate buffer the pKa value of H_2PO_4- is equal to 72 so that the buffer system is suitable for a pH range of 72-1 or from 62 to 82. 1 M NaCl 20 mM Na2HPO4 10 ACN in water pH 95 Extensive column cleaning as.
Choice of Weak Acid for a Buffer Which of these compounds would be the best buffer at pH 50. The kidneys provide extremely powerful buffers but are still the second line of defense because they are a great deal slower than the lungs and can take up to 2 days to correct changes in pH. The most important of these is undoubtedly the H 2 CO 3 HCO 3 pair but side chains of the amino acid histidine in the hemoglobin molecule also play a part.
Which of the following would make the best buffer solution at a pH of 85. Click here to get an answer to your question Which of these compounds would be the best buffer at ph 50. The acidic buffer contains a weak acid and its salt with a strong base eg.
Ie the more concentrated solutions can handle MORE of an added base andor acid. The pH of blood is controlled by the buffering action of several conjugate acid-base pairs. For example if you wanted to have a pH stay around 61 you would use a potato as a buffer.
Since the target pH of the buffer to be prepared is 50 the best buffer system to use will be the acetate buffer since 50 fall in the maximum buffering range for this buffer system. And if the ratio is more than 1 then the logarithmic part will be positive and the pH of the buffer will be more than the. We can use the pH of a liquid to determine whether it is an acid or a baseIt also is helpful in determining the buffering capacity of a buffer.
You must be examining the water since you are adding this to allow it to increase to 7. The key difference between pH and buffer is that the pH is a logarithmic scale whereas a buffer is an aqueous solution. A solution of HC04 and NaC04 C.
Formic acid leftmathrmp K_mathrma38right acetic acid leftmathrmp K_mathrma476right or ethylamine leftmathrmp K_mathrma90right Briefly justify your answer. Although it doesnt have the smallest pH range it does a good job of keeping the pH around 6 while aspirin or Bufferin keep the pH around 3. Which of these compounds would be the best buffer at pH 50.
They consist of an acid-base pair. Which of these compounds would be the best buffer at pH 50. Calculate the pH after adding 00050 mol of NaOH to 10 liter of the best buffer.
The next method would be to create a buffer for pH 4. Which of these compounds would be the best buffer at pH 50. Now if the ratio of is less than 1 then the logarithmic term will be negative.
The equation which determine the pH of a buffer solution is Heisenberg equation which is. Acetic acid and sodium acetate. Chemistry questions and answers.
Thus the pH will be less than the value of the acid. Potassium bitartrate is also named potassium hydrogen tartrate. You have a 2500-mL sample of 100 M acetic acid Ka 18 10-5.
A solution of HCN and NaCN d. A typical buffer is a mixture of ethanoic acid CH3COOH and sodium ethanoate CH3COONa. Formic acid pka 375 acetic acid pka 47.
Calculate the pH of the best buffer. If the pH of human blood for instance gets outside the range 72 to 76 the results are usually fatal. The pH scale generally ranges from 0 to 14.
Which of these compounds would be the best buffer at ph 50. None of these could make a buffer at that pH During a titration of a 100 ml solution of 15 M HNO2 using a 40 M NaOH. Calculate the pH after adding 00040 mol HCl to 10 liter of the best buffer.
A solution of HCOOH and NaHCOO b. Formic acid pKa 38 acetic acid pKa 476 or ethylamine pKa.

Pin By Bandalia Sarai On Titulos Bonitos Y Digitales Huruf Tulis Tangan Panduan Belajar Buku

4 Best Ph Balance Pills Ultimate Alkaline Supplements Ph Balance Hcg Diet Hcg Diet Plan

2 00 Ph Standard Buffer Solution 1000ml The Curated Chemical Collection Buffer Solution Solutions Chemistry Labs
Ph Buffers Acids And Bases Introduction To Chemistry

Buffer C Ph Controlled 500 Mg 120 Vegetarian Capsules By Country Life Natural Vitamins Vegetarian Organic Aloe Vera
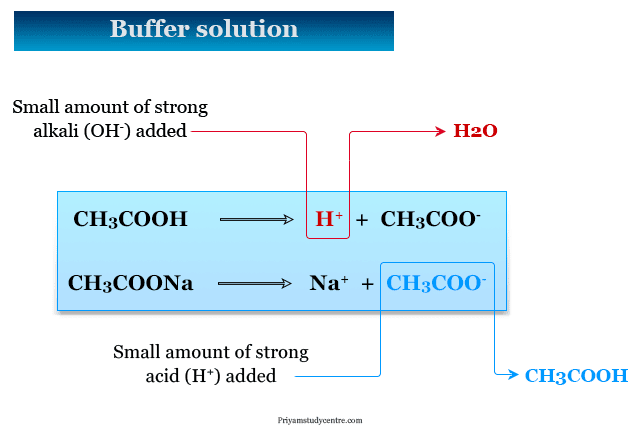
Buffer Solution Definition Types Uses
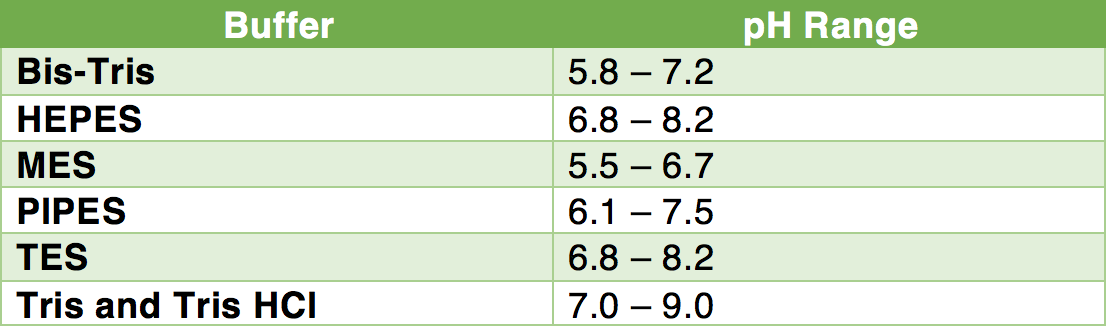
How To Prepare Your Most Frequently Used Buffers Goldbio

Aquaponics Ph Explained Finding The Perfect Balance Aquaponics System Aquaponics Diy Aquaponics

Bicarbonate Buffer Systems Chemistry Lessons Systems Biology Nursing School Studying

Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Physique Chimie Chimie

Standard Buffer Solution 500ml 10 0 Ph The Curated Chemical Collection In 2022 Buffer Solution Solutions Chemistry Labs

How To Prepare Buffer Solutions Teaching Chemistry Chemistry Lessons Buffer Solution

Standard Buffer Solution 1000ml 6 0 Ph The Curated Chemical Collection Buffer Solution Chemistry Labs Solutions

Ph Electrodos Potenciometros Conductimetros Buffers Peachimetros Conductividad Hammer






Comments
Post a Comment