Which of the Following Is Not a Strong Acid
CONDUCTOMETRIC TITRATION OF STRONG ACID AND STRONG BASE HCl vs NaOH AIM To determine the amount of strong acid HCl present in the given solution by conductometric titration using standard NaOH of 01N. A strong acid yields 100 or very nearly so of H 3 O and A-when the acid ionizes in water.
The reaction occurs at high sulfuric acid concentration 80 wt and low temperature eg.
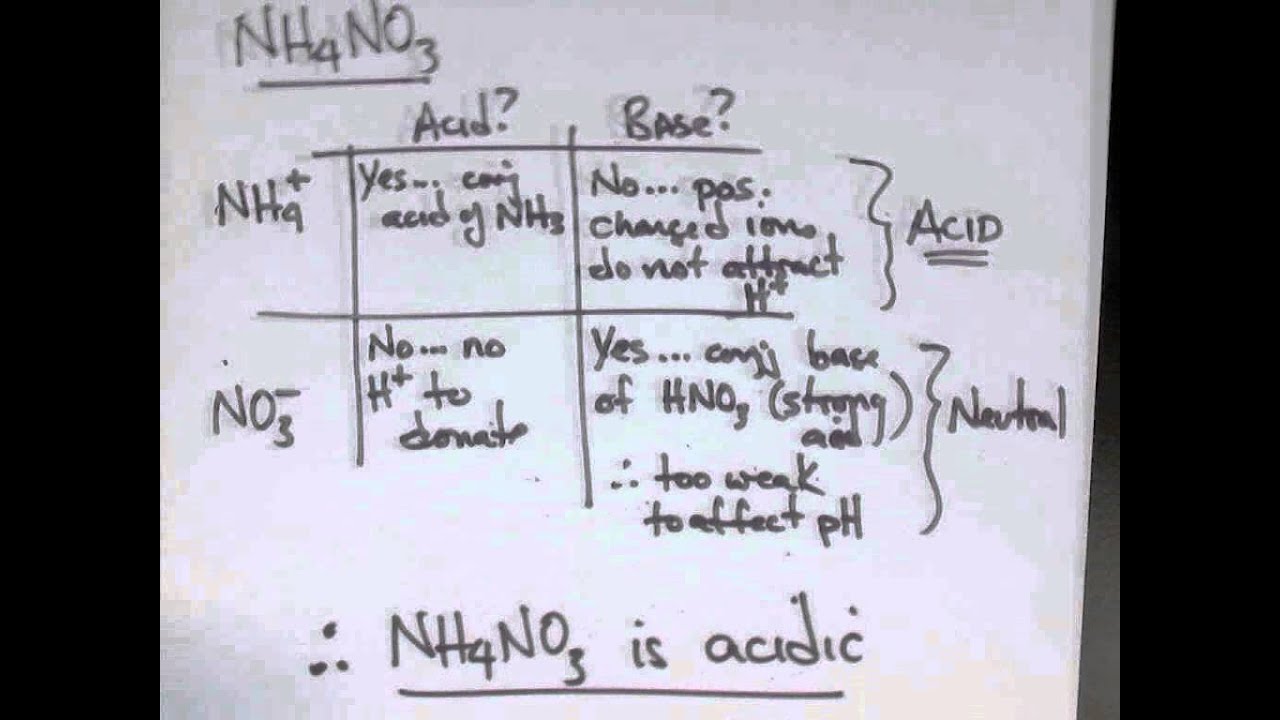
. The conjugate base of a strong acid is much weaker than water as a base. Materials used for acid piping have evolved with the design of acid plants over the years and with the introduction of new materials. Most problems asking for pH are for strong acids because they completely dissociate into their ions in water.
Classify each substance as a strong acid strong base weak acid or weak baseDrag the appropriate items to their respective binsLiOH HF H2SO4 CH3COOH HClO4 NaOH CaOH2 NH3 HBr HCOOHCsOH HNO2 HI HCN KOH CH32NH BaOH2 HNO3 HCl CH3NH2 Strong Acid Weak Acids Strong Bases Weak BasesPart BWhat salt is. The value of K a for acid is calculated from the following equation. For example in liquid ammonia acetic acid ionizes completely and may.
As the alkaline diet is predominantly made up of fruits vegetables and water with a distinct absence of fatty or sugary foods which are notoriously acidic it is also beneficial for weight loss. In contrast the right half of the spectrum below 2000 cm-1 normally contains many peaks of varying intensities many of which are not readily identifiable. Reaction of an acid with water is given by the following general expression.
Aerobic exercise for eight weeks provides protective effects towards liver and cardiometabolic health and adipose tissue. HAaq H 2 l H 3 Oaq A-aq Water is the base that reacts with the acid HA A-is the conjugate base of the acid HA and the hydronium ion is the conjugate acid of water. When a strong acid dissolves in water the acid reacts extensively with water to form H 3 O and A-ions.
In most applications strong acids are discussed in relation to water as a solvent. Weak acids on the other hand only partially dissociate so at equilibrium a solution contains both the weak acid and the ions into which it dissociates. However acidity and basicity have meaning in nonaqueous solvent.
The two most common materials used for the main acid circulating lines are castductile iron and alloy materials. As for weaker acids their relative. The main differences advantages and disadvantages are listed in the following table.
Two signals which can be seen clearly in this area is the carbonyl group which is a very strong peak around 1700 cm-1 and the C-O bond with can be one or two strong peaks around 1200 cm. As an ACID guarantee. Analyze the following ABG WARNING.
Schisandrin B mitigates hepatic steatosis and promotes fatty acid oxidation by inducing autophagy through AMPKmTOR signaling pathway. In the two-step strong-acid process separate reactors are used for the propylene-absorption phase and the hydrolysis of the sulfate esters. Only a small residual concentration of the HA molecules remains in solution The product of the concentrations of the H 3 O and A-ions is therefore much larger than the concentration of the HA molecules so K a.
The simplicity of this will depend on whether you have a strong acid or a weak acid. Since specific conductance of a solution is proportional to the. The following acids dissociate almost completely in water.
April 7 2022. The weak-acid process is conducted in a single step at lower acid concentration 6080 wt and higher temperature. Consistency is one of the four guarantees that define ACID transactions.
The increased intake of alkaline foods not only helps balance the stomachs acidity but it has also been shown to reduce the risk of inflammation following injury and infection. This does not guarantee correctness of the transaction in all ways the application programmer might have wanted that is the responsibility of application-level code but merely that any programming errors cannot result in the violation of any defined database constraints. PRINCIPLE Solution of electrolytes conducts electricity due to the presence of ions.
This site is not compatible with Internet Explorer 10 and lower and support for Internet Explorer 11 will be dropped very soon.

Welcome To Learnapchemistry Com Ap Chemistry Ap Chem Chemistry

Buffers And Henderson Hasselbalch Equation Chemistry Worksheets Chemistry Notes Teaching Chemistry

The Effect Of The Leaving Group On The Rate Of Sn2 Reactions Organic Chemistry Study Reactions Hydrogen Bond

Pin On Chemistry Step By Step Notes

Tretinoin And The Ordinary How To Put Together A Routine Tretinoin Tretinoin Before And After Skin Care

Neet Previous Year Question 2017 Chemistry Notes Chemistry Chemistry Class 12

Kangen Water 2 5 Strong Acidic Water Video Kangen Water Kangen Water

How To Build The Perfect Garage Gym Design In 2021 Best Home Gym Equipment Best Gym Equipment Garage Gym Design

Youtube Organic Chemistry Chemistry Organic Chem

What Makes A Good Leaving Group In Nucleophilic Substitution And Elimination Reactions Chemistry Help Chemistry Teaching Chemistry
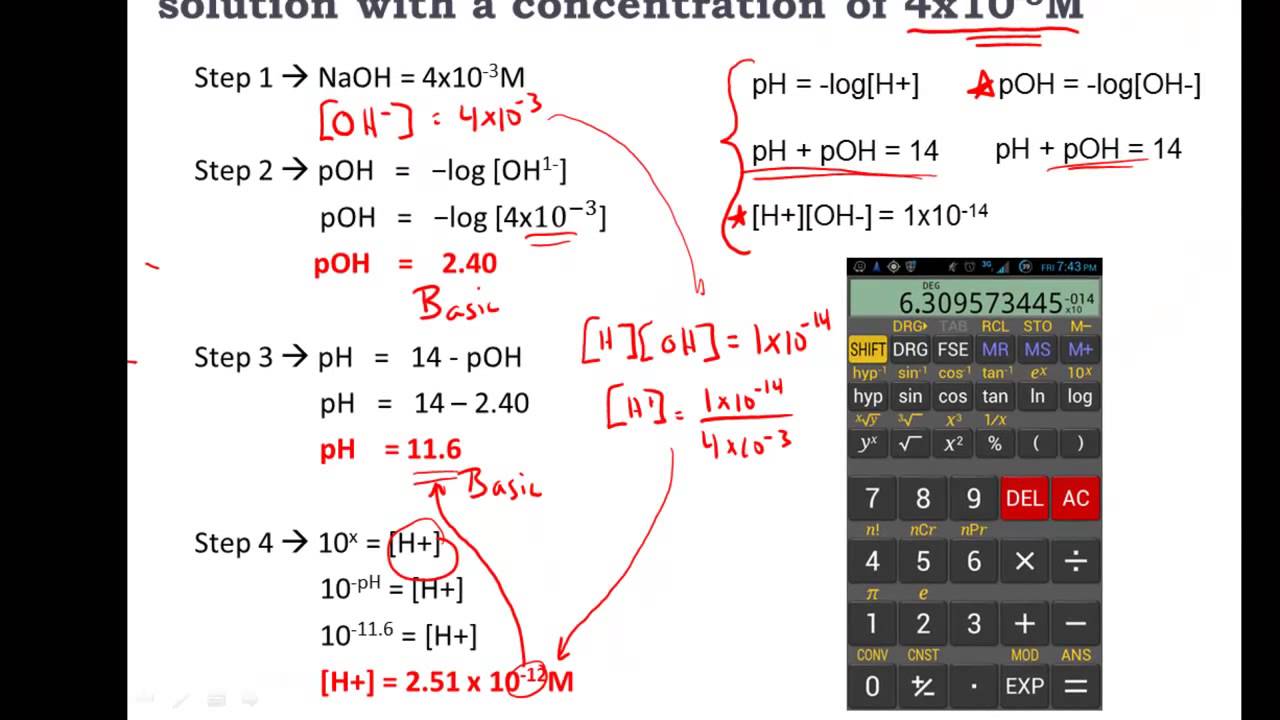








Comments
Post a Comment